Contract Imaging Services
Particle characterisation and high-speed imaging contract services and systems to enable actionable insight
Imaging Services at a Glance
Providing image-based services to industry and academia for over 20 years, Oxford Lasers have worked on a wide range of applications from drug delivery system characterisation through to particle sizing and velocity for agrochemical and fire suppression systems. We provide our customers with in-depth evaluation in high-speed imaging applications for actionable insight using our illumination and particle sizing systems.
Imaging Contract Services

In-House, On-Site, Rental or System Purchase?
We have dedicated in-house imaging laboratories. Ask if conducting services on-site at your organisation is an option. You may even consider the purchase of your own system for unlimited testing possibilities. Lets discuss what is best suited for you.
Inhaled Drug Delivery Characterisation
Providing analytical data to support the qualification and validation of spray devices. UKAS ISO17025:2017 process (accreditation no. 20625) to test pharmaceutical spray devices with high-speed image capture of spray plume (plume geometry and spray pattern).
Using our in-house imaging systems, we provide a wide range of qualitative and quantitative data on devices including high-speed imaging, spray pattern and plume geometry, event duration and velocity. Whether you are working on pressurised metered dose (pMDI) or breath actuated inhalers (BAI), intranasal or dry powder inhalers (DPI), soft mist inhalers (SMI) or the latest smart devices, we are able to provide rapid screening tests to bring your device to market faster. For breath actuated devices, our unique process allows us to capture data on the performance and behaviour of BAI, DPI and SMART inhaler devices under co-flow conditions.
Our Imaging team have a wealth of experience in image capture and analysis and would be happy to discuss how we align with regulatory requirements to conduct feasibility studies, process analysis or device characterisation. We can help.
High-Speed Imaging
High-speed imaging systems with short-pulse laser illumination for enhanced high-speed imaging performance.
Our equipment has been used on thousands high-speed imaging systems. Oxford Lasers combine high-speed cameras with state-of-the-art, short-pulsed diode lasers to offer one of the most powerful imaging systems to both academia and industry.
The short pulse capability of the lasers combined with their high frequency capacity enables the time resolved study of transient high-speed events. The short pulse effectively reduces the exposure time of the camera to nanoseconds and as a result motion blur is eliminated. Using our powerful laser illumination technology, we support our customers with imaging processes that have previously been left unseen.

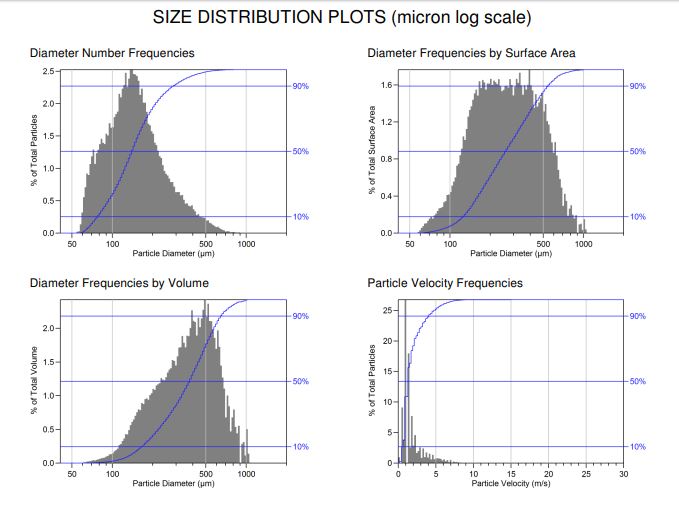
Particle Size and Velocity Measurements
Velocity measurement of particles, droplets or bubbles in real-time with image-based analysis made possible with VisiSize tools
For when you need to know how fast a particle or spray droplet is moving, VisiSize features robust software with particle tracking. This produces size-velocity correlations which allows you to see the velocity of the droplets or particles at the same time as measuring the size. Velocity measurements are at the core of calculating a spray or particle flow and are a valuable tool in understanding the nature of your system.
Particle Size vs Particle Velocity
Our powerful sizing software allow simultaneous measurement of particle size and velocity. A visualization of the relationship between size and velocity enables rapid process optimization, highlighting the effects of parameters under test (such as nozzle type) on the flow rate; and pressure on the droplet size/velocity distribution.
We provide analysis services with actionable insights into your product and process development.

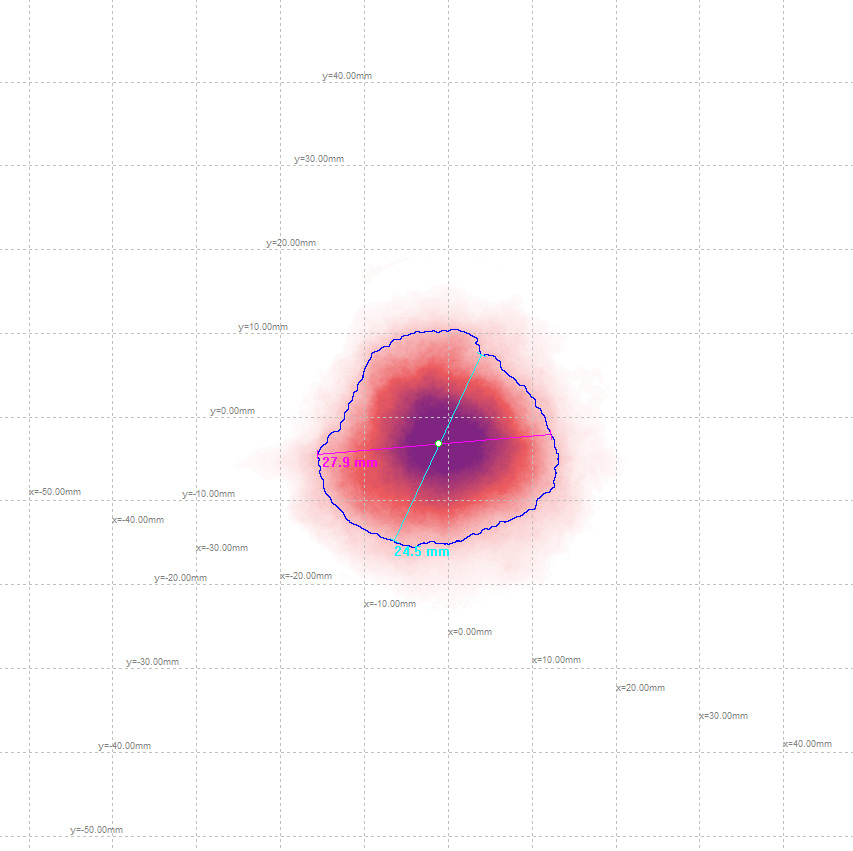
Spray Analysis: Spray Pattern and Plume Geometry Measurement
Our in-house spray pattern measurement systems are used for characterisation of many types of sprays in various sectors including inhaled drug delivery devices in pharmaceutical and medical device development, chemicals in agrochemical and nozzle development, cooking oils in the food and drink sector, fuel injectors in the automotive industry and domestic aerosols for consumer goods companies.
Oxford Lasers’ imaging systems use a high-speed camera and powerful laser illumination to capture high-quality, slow-motion imaging. This reveals the dynamic performance of products that would otherwise be unseen and therefore unknown. Our software analyses the highly detailed images by measuring key parameters such as the dimensions of the spray pattern, cone angle (plume geometry) and event duration. Further analyses permit droplet size and velocity measurements including velocity profiles of each event. There is powerful information resulting from high-quality imaging to pinpoint product performance and further its development.

Bright-Event Imaging
Using laser illumination to eliminate glare enabling a clear image of a bright event.
Whether you want to see a still image or capture a video of a bright event process, seeing exactly what is happening at the heart of the glare is possible with the use of our FireFLY and FireBIRD laser illumination products. The laser’s specific, single wavelength enables excess light from bright events to be filtered out, providing greater insight into bright events such as welding and ballistics.
Whether you wish to capture images through flames, welding arcs or explosions, when a bright event is imaged with a normal camera, the high brightness saturates the camera and the detail cannot be seen; However, our laser illumination systems eliminate the glare to produce clear imaging of the event. We’ll work with you to reveal your processes with advanced laser imaging services.

Published Research Using Oxford Lasers Imaging Products or Services
Local dynamics of pharma powder fluidization using high speed imaging and PIV | K. Elserfy, S. Cheng, H-K. Chan, A. Kourmatzis, Local dynamics of pharmaceutical powder fluidization using high speed long distance microscopy and particle image velocimetry, Experimental Thermal and Fluid Science, Volume 124, 2021, 110367.
https://doi.org/10.1016/j.expthermflusci.2021.110367 (external link, opens new window)
Effect of an upstream grid on the fluidization of pharmaceutical carrier powders | 2020 K. Elserfy, A. Kourmatzis, H.-K. Chan, R. Walenga, S. Cheng, Effect of an upstream grid on the fluidization of pharmaceutical carrier powders,International Journal of Pharmaceutics, Volume 578, 2020.
https://doi.org/10.1016/j.ijpharm.2020.119079 (external link, opens new window)
High-Speed Laser Image Analysis of Plume Angles for Pressurised Metered Dose Inhalers: The Effect of Nozzle Geometry
Chen, Y., Young, P.M., Murphy, S. et al. High-Speed Laser Image Analysis of Plume Angles for Pressurised Metered Dose Inhalers: The Effect of Nozzle Geometry. AAPS PharmSciTech 18, 782–789 (2017). https://doi.org/10.1208/s12249-016-0564-5
Acoustic methodology for measuring discharge rate, and predicting spray velocity effects on potential lung deposition
A Systematic Approach in the Development of the Morphologically-Directed Raman Spectroscopy Methodology for Characterizing Nasal Suspension Drug Products
Farias, G., Shur, J., Price, R. et al. A Systematic Approach in the Development of the Morphologically-Directed Raman Spectroscopy Methodology for Characterizing Nasal Suspension Drug Products. AAPS J 23, 73 (2021). https://doi.org/10.1208/s12248-021-00605-w
Imaging Systems
We offer our imaging systems for customers to perform their own analyses with our VisiSize particle characterisation series and our FireFLY and FireBIRD laser illumination systems for high-speed imaging applications. High-intensity light sources at ultra-fast speeds enable real-time visualisation of information. From droplet, particle and bubble size measurements for spray analysis (speed, shape and velocity) to imaging of bright and energetic events – Oxford Lasers has a range of products to meet your project requirements.
Find out more about our high-performance systems.
Contact Us
For more information on how we might be able to help you and learn more about our products and services, submit an enquiry below.